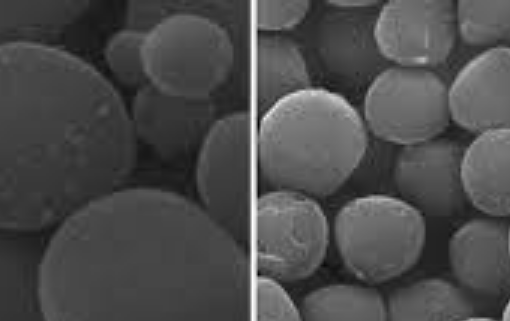
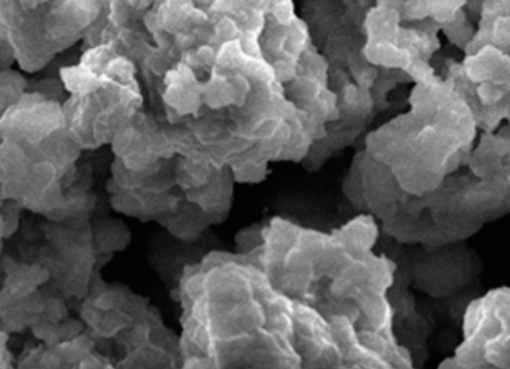
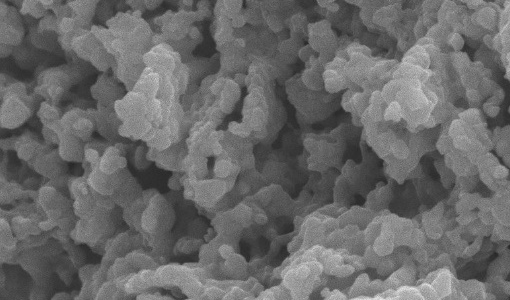
Iron open metal site in metal-organic frameworks, MIL-88A and MIL-127(Fe), was tested to investigate its performance on CO2 separation from a binary mixture CO2/N2 under simulated post-combustion system. The sorption experiment tests were conducted via a fixed-bed reactor with different temperatures ranging between 30°C and 60°C at atmospheric pressure. Maximum CO2 sorption capacity was observed at low temperature of 30°C, where 5.24 mmol could be adsorbed by one gram of MIL-127(Fe) and 4.95 mmol CO2 could be adsorbed by one gram of MIL-88A. The presence of moisture in the gas mixture affected CO2 sorption capacity by reducing CO2 capacity as high as 0.5 mmol CO2 per one gram sorbent. In this work, a dynamic breakthrough model was applied to investigate adsorption behavior of the metal-organic frameworks (MOFs) and of the system. The predicted model showed good agreement with experimental results; the Langmuir isotherm model fitted well with our sorption system, suggesting one molecule of CO2 is held at localized sites of the MOFs, which plausibly be at unsaturated Fe2+ metal ion site. Rate of sorption was found to increase with increasing sorption temperature for either intra-particle diffusivity or external mass transfer. Our study suggests that the MOFs containing Fe open metal sites offer an outstanding prospective for CO2 sorption and both MIL-88A and MIL-127(Fe) are good representatives applied for post-combustion CO2 capture.
Effect of Fe open metal site in metal-organic frameworks on post-combustion CO2 capture performance

Related tags :
S. Wongsakulphasatch
Related Articles
August 31, 20200
SDS modified mesoporous silica MCM-41 for the adsorption of Cu2+, Cd2+, Zn2+ from aqueous systems
SDS-functionalized mesoporous silica MCM-41, was synthesized and used as adsorbent for remediating toxic metal contamination. The new material was applied to capture the heavy metal ions Cu2+, Cd2+, a
Read More
October 30, 20140
The adsorption aspect of Cu2 + and Zn2 + on MCM-41 and SDS-modified MCM-41
This study performed both macroscopic and microscopic investigation to determine the interaction mechanism for heavy metal adsorption on functionalized MCM-41 mesoporous silica. Hybrid sorbent materia
Read More
May 3, 20190
Influence of CaO precursor on CO2 capture performance and sorption-enhanced steam ethanol reforming
This work presents the effect of calcium and carbonate precursors on properties of CaCO3. The synthetic CaCO3 samples were transformed into CaO and tested their application to high-temperature CO2 cap
Read More
October 30, 20210
Dr. S. Wongsakulphasatch
Heavy metal adsorption by porous solids
Materials and systems for CO2 capture
Hydrogen production
Colloid and interface science
Process Integration
Analytical Science
Dr. Won
Read More
May 4, 20210
Optimisation of a Sorption-Enhanced Chemical Looping Steam Methane Reforming Process
An intensified hydrogen production steam reforming process named ‘Sorption-Enhanced Chemical Looping Steam Methane Reforming’ (SE-CL-SMR) was studied. Aspen Plus was used to carry out a thermodynamic
Read More
May 4, 20200
Bi-metallic CuO-NiO based multifunctional material for hydrogen production from sorption-enhanced chemical looping autothermal reforming of ethanol
Bi-metallic CuO-NiO based multifunctional materials were developed and employed for H2 production via sorption-enhanced chemical looping autothermal reforming (SE-CLAR) of ethanol. The effects of addi
Read More
May 4, 20130
Comparative study of fuel gas production for SOFC from steam and supercritical-water reforming of bioethanol
Theoretical study of fuel gas (H2 & CO) production for SOFC from bioethanol was carried out to compare performances between two reforming technologies, including steam reforming (SR) and supercrit
Read More
June 4, 20160
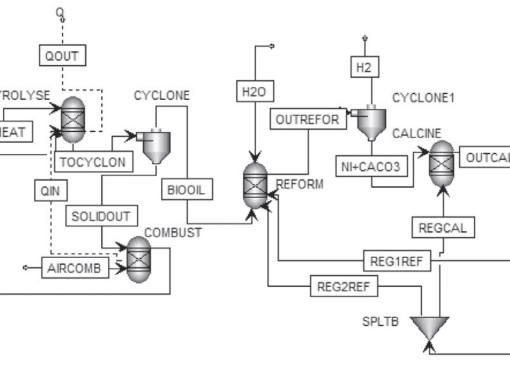
Performance evaluation of sorption enhanced chemical-looping reforming for hydrogen production from biomass with modification of catalyst and sorbent regeneration
Process simulation of sorption enhanced chemical-looping reforming for hydrogen production from biomass was investigated. Corn stover was converted to bio-oil via pyrolysis prior to sorption enhanced
Read More