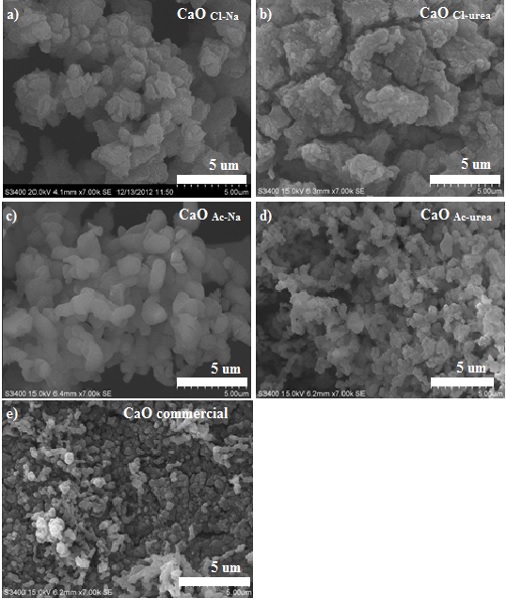
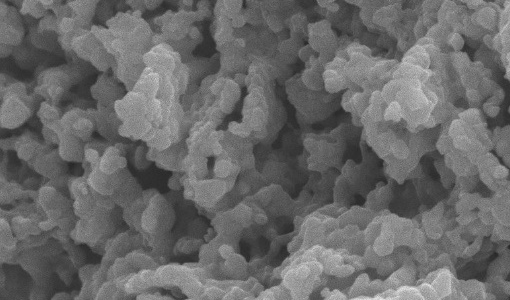
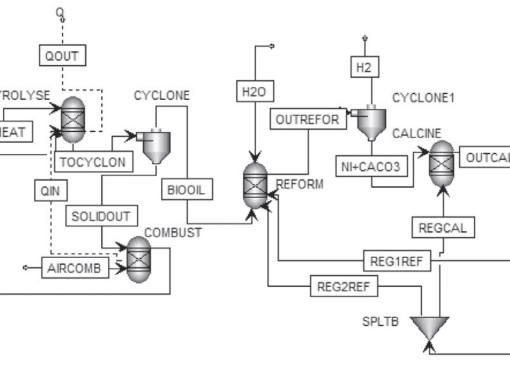
This work presents the effect of calcium and carbonate precursors on properties of CaCO3. The synthetic CaCO3 samples were transformed into CaO and tested their application to high-temperature CO2 capture. Four different sorbent precursors were investigated in this work, including calcium chloride (CaCl2) and calcium acetate (CaAc2) as calcium precursor, and sodium carbonate (Na2CO3) and urea (CO(NH2)2) as carbonate precursor. The results show that both calcium and carbonate precursors affect morphologies of CaCO3; CaCO3,Cl-Urea, and CaCO3,Cl-Na have calcite phase, whereas mixed-phases of calcite (30%) and vaterite (70%) are observed with CaCO3,Ac-Na, and aragonite is found with CaCO3,Ac-Urea. CaCO3,Cl-Na exhibits small cubic (rhombohedral) particle, CaCO3,Cl-Urea possesses spherical particle with rough surface, CaCO3,Ac-Na has spherical-like morphology with smooth surface, and CaCO3,Ac-Urea possesses aggregated form of CaCO3 particles. For application to CO2 capture, CaO derived from CaCO3,Ac-Urea provides the highest CaO conversion of 80% at 700 C. The synthetic CaO-based sorbents were further incorporated with nickel oxide to form one-body bi-functional catalysts for H2 production from sorption-enhanced steam ethanol reforming. The results show that 87% H2 purity could be obtained with pre-breakthrough period of 60 min. Sorbent reactivity can be maintained the production of H2 for at least 10 consecutive cycle tests.