Whey proteins are an important class of biopolymer molecules that are mainly globular and heat sensitive. Under conducive conditions, they undergo molecule unfolding and further self-assembly to produce nano-structured fine-stranded gels or particulate aggregates via non-covalent interactions. Self-assembly is a simple and efficient pathway to the construction of large and complex structures, and is a key process in nature. This phenomenon, exploited in a rational way is a promising route to the development of novel materials with tailored properties. However, the prediction and rational manipulation of the macroscopic properties of such supramolecules requires a mechanistic understanding of the microstructure-properties-aggregation mechanism relationship.
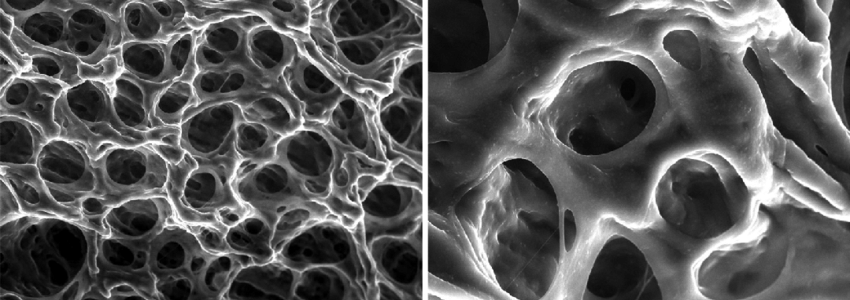
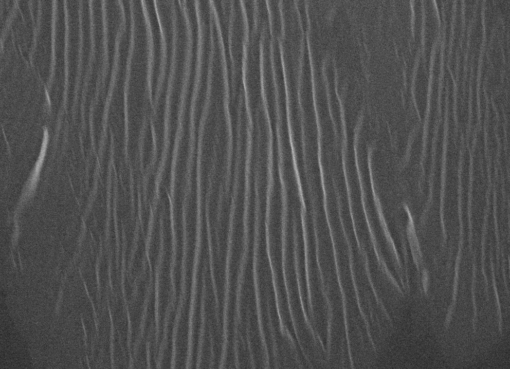
In this research an attempt is made to gain a mechanistic insight into the aforementioned phenomena, combining structural chemistry, biophysics, engineering and a wide range of measurement techniques at a variety of both length and time scales, using β -lactoglobulin ( β -Lg) as a model protein. The aggregation phenomenon is investigated by examining independently the effect of protein charge and charge shielding on the thermodynamics, kinetics and the structural transitions across the heat-induced aggregation using micro differential scanning calorimetry (micro DSC), rheology and quantitative Fourier transform infrared spectroscopy (FTIR) respectively. The rheological properties, internal structure and microstructure of the resultant aggregates are investigated by rheology, polarized light microscopy, environmental scanning electron microscopy (ESEM), and transmission electron microscopy (TEM). Because of the novel approach used in sample preparation and reproducible experimental conditions, this work has allowed the microstructure of aggregates to be correlated with their viscoelastic behaviour and aggregation dynamics conclusively.
The heat-induced aggregation of β -Lg arises from a non-reversible two-step conformational transition, which is strongly modulated by pH and ionic strength (RS).
The first step is comprised of an initial dimer dissociation process followed by a non-spontaneous molecular unfolding where an unfolded intermediate is transiently produced. In the second step, the transient intermediate spontaneously self-assembles and aggregates via non-covalent interactions driven by the hydrophobic effect. The heat-induced molecular unfolding is endothermic while the self-assembly and further aggregation process is reflected at the macroscopic level by a liquid-solid transition (LST). Five kinds of structures are obtained upon heat-induced aggregation of β -Lg in aqueous solutions depending on pH and ionic strength: A transparent viscous liquid comprised of amyloid fibrils stacked side by side into thicker fibrils at pH 7.1, transparent viscoelastic gels composed of a non-covalently cross-linked
amyloid fibrils network at pH≤3.6 or RS in the range 0.016 to 0.048, turbid particulate aggregates comprised of spherical-like particles at the isoelectric point, turbid particulate aggregates comprised of fractals at pH 4.2 or RS≥0.08 and an intermediate opaque gel comprised of an amyloid fibrils network that experiences syneresis at RS=0.064. The rheological behaviour of fine-stranded gels is dictated by the diameter of fibrils and the distance between web nodes.