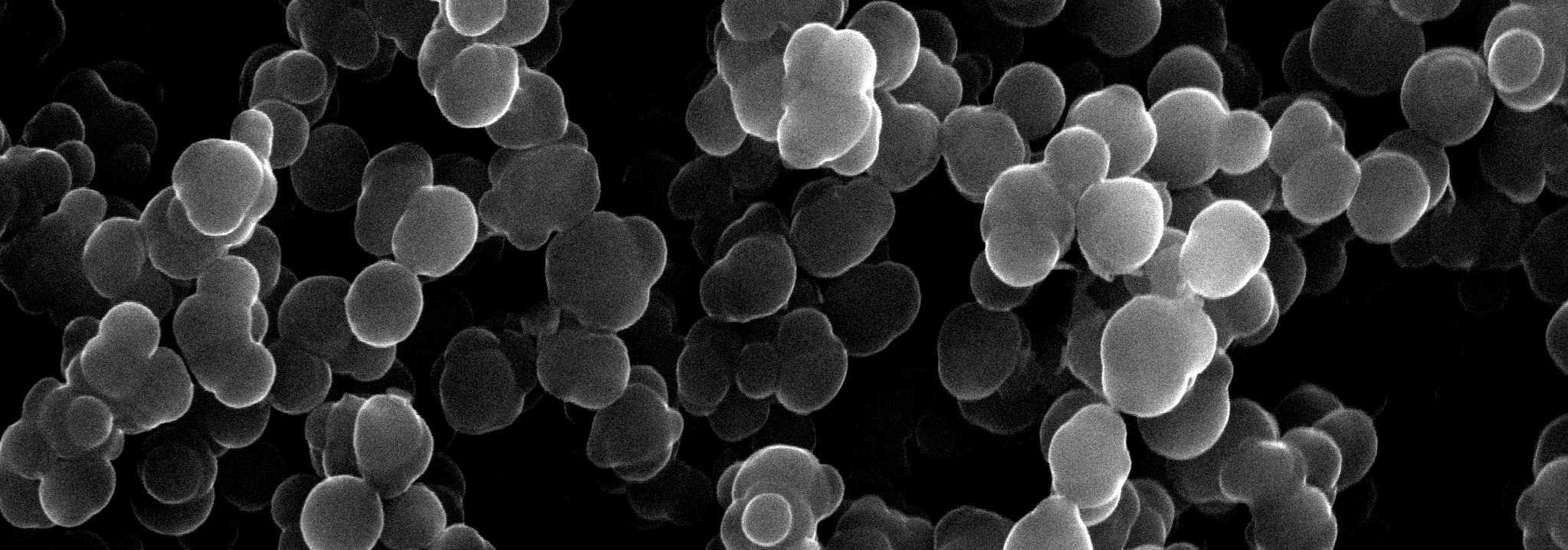
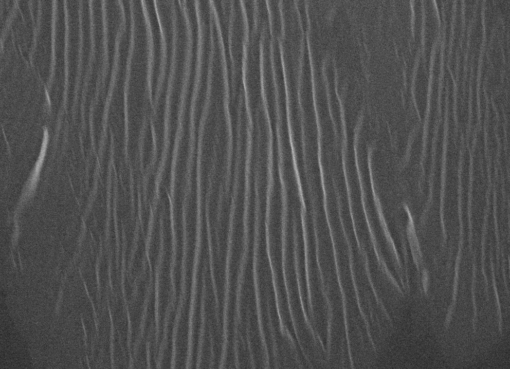
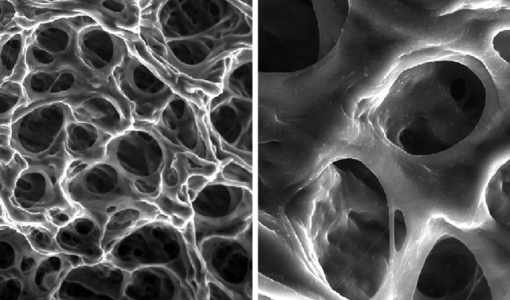
The microstructure-properties-aggregation mechanism of heat-induced aggregates of β-Lactoglobulin (β-Lg) and its response to pH and ionic strength modulation have been investigated by environmental scanning electron microscopy (ESEM) and rheology. A novel procedure of sample preparation presented here allowed a systematic and correlated insight into the microstructure-property-aggregation mechanism relationship. Five kinds of structures were obtained upon β-Lg heat induced self-assembly depending on pH and ionic strength: 1) A transparent viscous liquid comprised of unbranched amyloid-like fibrillar structures at pH 7.1 and zero ionic strength; 2) Transparent gels composed of fibrillar network at pH 3.6 with zero ionic strength or at ionic strength, RS from 0.016 to 0.048 with neutral pH; 3) Turbid particulate aggregates comprised of spherical-like clusters at isoelectric point; 4) Turbid particulate aggregates comprised of amorphous clusters at pH 4.2 or at ionic strength, RS 0.08 and 5) An intermediate opaque gel comprised of a fibrillar network that experiences syneresis at ionic strength RS=0.064. The cured gel obtained at pH 7.1 and zero ionic strength is a structured liquid comprised of a bundle of unconnected amyloid-like fibrils with viscoelastic behaviour dominated by viscous components. The liquid-solid transition (LST) and long relaxation mode experienced by this sample under heat-induced aggregation arises from the motion of entire fibrils and entanglement effects. The microstructure of fibrillar networks has similarities with amyloid fibrils and cross-linked chemical gels and consists of colloidal crosslinking fibrils dispersed within the aqueous solution in interpenetrating three-dimensional networks. Ionic strength (NaCl) and low pH (HCl) have an independent and qualitatively different fibril crosslinking effect on heat induced β-Lg fibrillisation. Fibrillisation, however is overruled when β-Lg molecule has roughly no net charge at pH close to isoelectric point or at ionic strength RS=0.08 and above with neutral pH. At these conditions, the heat induced transient unfolded intermediate collapse into particulate aggregates driven by a stronger hydrophobic effect and an overruled hydrophilic effect over polar residues, which is reflected in faster kinetics 1 and modified residue solvation patterns. The microstructure of fibrillar networks dictates the viscoelastic behaviour of crosslinking gels on the macroscopic level as a function of fibrils diameter and the distance between nodes. Ionic strength induces far superior rigidity on gels compared with low pH at the conditions investigated. That is explained by thicker fibrils and shorter distances between nodes as ionic strength increases, which can be ascribed to fibrils arranging laterally into thicker bundles induced by a stronger hydrophobic effect as ionic strength increases. The fibrillar network formed at ionic strength, RS=0.064 experiences syneresis and could be an intermediate structure between a colloidal gel network and an insoluble particulate aggregate. The turbid spherical-like or amorphous particulate aggregates produced at pH close to the isoelectric point and at high ionic strength (RS 0.08) do not bind the solvent and should not be considered as colloidal crosslinking gels.