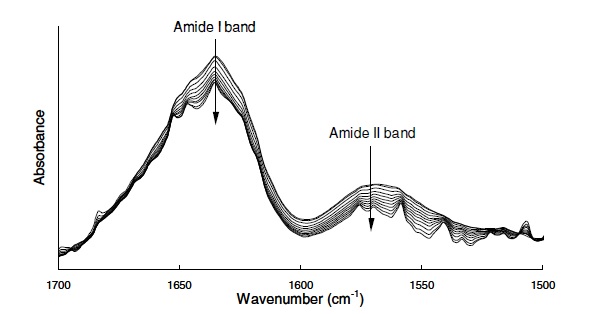
The conformational transition across the heat-induced aggregation of β-lactoglobulin ( β-Lg) in D2O and its modulation to pD and ionic strength has been investigated by quantitative Fourier transform infrared spectroscopy (FTIR) and micro differential scanning calorimetry (microDSC). Calorimetric results reveal that the substitution of water by heavy water as a solvent does not change the way protein charge and electrostatic interactions control molecular unfolding and the self-assembly route. From IR, it is evident the molecular structure of β-Lg in D2O is comprised of thirteen structural components. A conformational transition is induced by pD and ionic strength during incubation which is assigned to molecule unfolding and dimer splitting. Evidence shown here indicates that the endothermic effect observed in calorimetric studies is attributed to molecule unfolding prior to self-assembly and further aggregation processes. Heat-induced aggregation of β-Lg in D2O solutions is characterized by a structural transition and onset, which are modulated by pD and ionic strength. Prior to molecule unfolding, minor changes in secondary structure were observed from IR, which are assigned to dimer splitting. Structural changes observed indicate that molecule unfolding occurs via the splitting of hydrogen-bonded-strands connected by loops, which then become exposed to solvent. Subsequently, the molecules spontaneously self-assemble and undergo further aggregation processes driven by the hydrophobic effect. Onset temperature, conformational transition, extent and kinetics of unfolding and self-assembly route are modulated by pD and ionic strength and are different for each condition investigated.