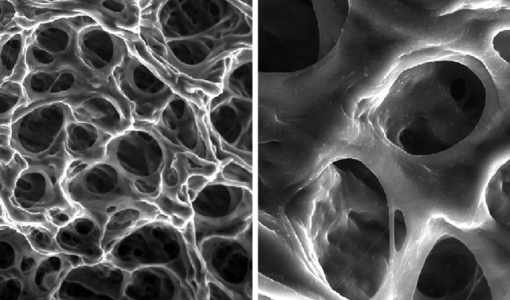
Heat induced aggregation of β-lactoglobulin ( β-Lg) and its modulation to pH and ionic strength has been investigated by micro differential scanning calorimetry (microDSC) and rheology. A novel procedure on sample preparation was adopted to achieve a reproducible and correlated insight into β-Lg aggregation dynamics. The heat-induced aggregation of β-Lg proved to be a non-reversible two-step process modulated by pH and ionic strength with different mechanisms. The first step is comprised of an initial dimerization-dissociation process followed by a non-spontaneous energy activated molecule unfolding where an intermediate with different level of unfolding is transiently produced. In the second step, once the transient intermediate reaches its maximum level of unfolding, it spontaneously self-assembles and aggregates driven by electrostatic interactions and hydrophobic effect. Initially, quaternary contacts induced by a dimer-monomer equilibrium dictate the rheological behaviour of solutions of β-Lg at 20 °C, with G’ prevalent over G” at pH 7.1 and 5.2 (equilibrium shifted toward dimeric aggregates) or a G” prevalent over G’ at pH 4.2 and lower (equilibrium shifted towards monomers). At neutral pH, ionic strength keeps equilibrium towards dimeric aggregates, which is reflected in rheological behaviour dominated by elastic components. Protein unfolding is characterised by a large endothermic effect with onset in the range from 67 to 81 °C and delta enthalpy, ΔH in the range from 11.8 to 18 J g -1 . Unfolding is a temperature dependant activated process where protein charge and hydrophobic effect dictate the level of unfolding of the transient intermediate and thus the energy requirement. At pH 3.6 and lower, where aggregates structure is comprised of fibrillar networks, liquid-solid transition (LST) is characterised by a short relaxation mode, whereas above pH 3.6, LST is characterised by a long relaxation mode ascribed to the motion of fractals at isoelectric point or amyloid-like fibrillar structures at pH 7.1. Unfolding is modulated by ionic strength with a different mechanism because at neutral pH and the range of ionic strength investigated, the quaternary structure is predominantly dimeric and protein charge is only slightly modified. ΔH slightly decreases (12.4 to 11.4 J g -1 ) as the ionic strength; RS increases from 0 to 0.032. At RS=0.048 and above, ΔH is almost constant (12.4 to 12.8 J g -1 ) indicating a similar unfolded intermediate precursor in the particulate aggregates encountered at high ionic strength. At ionic strength RS=0.064 and higher, an endothermic effect with double peaks are encountered likely because ionic strength makes hydrophobic effect strong enough to force unfolding to occur in two steps with the second step activated at higher temperature. Increasing values of onset/peak and faster kinetics /lower LST point as ionic strength increases are observed, which is a behaviour solely ascribed to the hydrophobic effect. It arises from the interaction between ionic strength and molecule core hydrophobicity, which produces a dual effect: a stronger molecule unfolding inhibitor and self-assembly catalyst as ionic strength increases. The resultant structures are stable aggregated macromolecules with different microstructure dictated by the unfolded intermediate, preferred interactions during self assembly process and kinetics. The description of the whole aggregation mechanism and resultant structures by empirical models as have been suggested seems thus unrealistic.
Dynamics of heat induced self-assembly of nanomaterials: A correlated insight into the aggregation mechanism of β-Lg and its modulation to pH and ionic strength
Related tags :
S. Garcia
Related Articles
March 28, 20080
Amyloid fibrils as self-assembled nanomaterials: An insight into biopolymer gelation
Whey proteins are an important class of biopolymer molecules that are mainly globular and heat sensitive. Under conducive conditions, they undergo molecule unfolding and further self-assembly to produ
Read More
November 2, 20210
Dr. S. Garcia
Analytical Science
Molecular Medicine
Modeling & Simulation
Oil & Gas
Dr. Garcia holds a Doctorate in Chemical Engineering and Analytical Sciences from The University of Ma
Read More
December 3, 20070
Heat induced self-assembled β-Lg gels and fractals: The microstructure-properties-aggregation mechanism relationship and its response to pH and ionic strength modulation
The microstructure-properties-aggregation mechanism of heat-induced aggregates of β-Lactoglobulin (β-Lg) and its response to pH and ionic strength modulation have been investigated by environmental sc
Read More
July 15, 20160
Assessment of Fuel Oil Availability
MARPOL Annex VI requires all ships to use fuels with a sulphur content of 0.50% m/m from 1 January 2020 onwards (in emission control areas, other limits apply). The implementation date is subject to a
Read More
December 3, 20070
Dynamics of heat-induced self-assembly of nanomaterials: A mechanistic insight into the structural transition across the aggregation of β-Lg gels and fractals
The conformational transition across the heat-induced aggregation of β-lactoglobulin ( β-Lg) in D2O and its modulation to pD and ionic strength has been investigated by quantitative Fourier transform
Read More